Plastics
Hard Polyurethane (PUR hard)
General information
Description
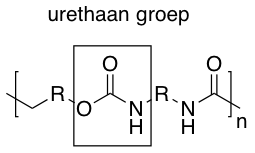
Hard polyurethanes are plastics that can have a variety of chemical compositions, the two main groups are PUR esters and PUR ethers. Their appearance ranges from clear and transparent to opaque. They do not have a characteristic sound or touch. However, sometimes they have a sweet chemical smell.History
The first polyurethanes were developed in 1937. In 1957 polyurethane foam in a spray can was introduced. First only PUR ester, from 1960 onwards also PUR ether.Production, Application, Appearance
The best known and most produced form of PUR is foam. The hard foam is often used as insulation material from a spray can. It is a material that artists can make themselves by mixing two components. Therefore, it is used frequently in the art world.Properties
Material properties
ThermosetDensity: 1.1-1.25 g/cm3; foam: ~0.04-0.45 g/cm3 Melting point: 141-157°C
Melting point: 141-157°C
Glass transition temperature: -60 to -19°C
Identification properties
Cell structure (foam): closedSmell: can have a sweet chemical smell
Touch: no characteristic touch
Sound: no characteristic sound
UV-radiation (when clear): fluoresces blue clearly
Polarizing filters (when clear): does (not) give a (colour) pattern
Degradation
Process
Photo-oxidation, hydrolysis.Details
PUR is considered a problem plastic. Hard PUR may give less problems than the other types, it still degrades. Due to its open cell structure hard PUR foam degrades not only on the surface but into the core as well.Symptoms
Discolouration; crumbling; surface becomes matte; loss of mechanical properties resulting in tears and fractures; surface becomes tacky; efflorescence of white bloom; disintegration.Susceptibility
The susceptibility of flexible polyurethane depends on whether it is a PUR ester or PUR ether. PUR esters are highly susceptible for hydrolysis, at high RH the surface becomes tacky. Ethers have a high susceptibility for photo-oxidation (UV and light) which causes discolouration and crumbling. See appendix for a chemical test to distinguish the two.Ester:
UV-radiation: Medium
Light: Medium
Oxygen/Ozone: Low
Temp: Low
RH: High (setpoint)
Ether:
UV-radiation: High
Light: High
Oxygen/Ozone: Medium
Temp: Low
RH: Medium
Preventive conservation
Recommendations
Ester:UV-RADIATION: keep below 75 µW/lm UV filter for daylight and fluorescent light - reduce intensity
LIGHT: 1 slight change in approx. 30 Mlx.h Moderate light dose - control intensity and exposure time
OXYGEN / OZONE: ambient conditions
TEMP: common indoor conditions 10-30°C; a lower temperature will slow down hydrolysis
RH: 10-30% RH fluctuations: within bandwidth
—
Ether:
UV-RADIATION: keep below 10 µW/lm Exclude UV with filters or no-UV light source
LIGHT: 1 slight change in approx. 1 Mlx.h Limit light dose by reducing intensity and exposure time
OXYGEN / OZONE: lower temperature slows down oxidation
TEMP: common indoor conditions 10-30°C
RH: common museum conditions 40-60% RH fluctuations: setpoint ±10% or ±5% when allowing seasonal fluctations between 35-65%
Other names
- PU-schuim
- Kapaline
Am I dealing with...
TAGS
- Closed cell structure
- Sticky
- Crumbly
- Skin
- Hard
- Sprayed form