Plastics
Cellulose nitrate (CN)
General information
Description
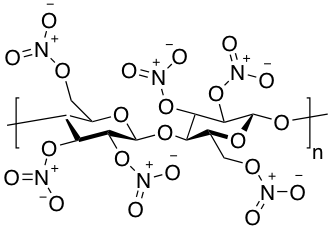
Cellulose nitrate (CN) is an early plastic, a half-synthetic material. Is was used as an imitation of natural materials such as tortoiseshell and ivory. Camphor was added as stabilizer and plasticizer which gives CN its characteristic smell. The material is highly flammable and susceptible for degradation. The sugar-like degradation pattern is typical for CN.History
Cellulose nitrate was first discovered in 1832 but it was not until 1855 that it could be stabilised with camphor and came into production under the name Celluloid. Due to its high flammability it was gradually replaced by cellulose acetate and went out of use after 1950.Production, Application, Appearance
CN film was used as base material for photographic and motion picture film and animation cells. After 1950 it was replaced by the less flammable cellulose acetate which was thought to be more stable. Rigid CN can be transparent and opaque. It was used mainly as imitation of tortoise and ivory for trays, boxes, and fashion accessories. A well-known application is in sleeves and collars. Nowadays CN is used only for the production of table tennis balls.Properties
Material properties
ThermoplasticDensity: 1.35-1.40 g/cm3
Glass transition temperature: 53-66°C
Identification properties
Cell structure (foam): not applicableSmell: camphor / mothballs
Touch: no characteristic touch
Sound: no characteristic sound
UV-radiation (when clear): fluoresces distinctly yellow
Polarizing filters (for clear CA): does not produce a colour pattern
Degradation
Process
Upon hydrolysis nitric acid is produced which accelerates further degradation (auto-catalysis).Details
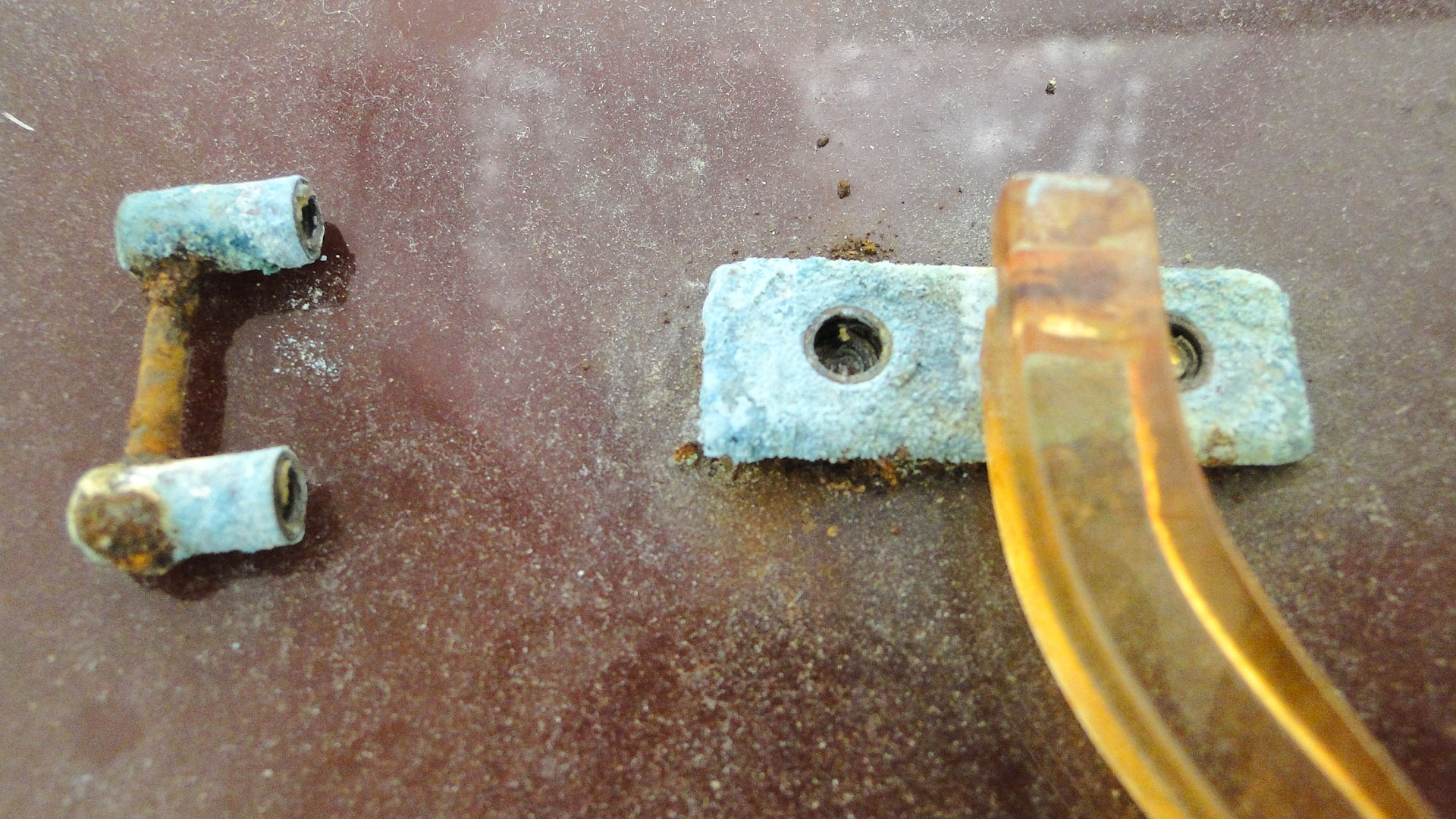
CN is a problem plastic owing to its auto-catalytic degradation.Symptoms
Yellowing; craquelure; sugar-like degradation; acid smell; loss of mechanical properties resulting in tears and fractures; deformation (warping); surface becomes moist (weeping); formation of white crystals on the surface.Susceptibility
UV-radiation: MediumLight: Medium
Oxygen/Ozone: Low
Temp: High
RH: Highly sensitive to hydrolysis
Preventive conservation
Recommendations
UV-RADIATION: keep below 75 µW/lm UV filter for daylight and fluorescent light - reduce intensityLIGHT: 1 slight change in approx. 30 Mlx.h Moderate light dose - control intensity and exposure time
OXYGEN / OZONE: ambient conditions
TEMP: keep cool (5°C) or cold (-20°C) to slow down auto-catalytic degradation
RH: 20-30% RH fluctuations: keep constant - setpoint ±5%
—
Note: the emitted nitric acid corrodes metals and can affect other objects. Store CN as cold as possible. Do not seal packaging. Ensure good ventilation. Keep away from susceptible objects. Acid absorbing material such as activated charcoal or buffered paper can be placed close to the object.
Other names
- Celluloid
- Xylonite
- Nitrocellulose
- Parkesine